
The bonding (attracting) MO is full, and the antibonding (repulsing) MO is empty. Antibonding MO’s are the consequence of destructive interference which results in a repulsive interaction and a region of zero electron density between the nuclei (in other words, a node).įollowing the same aufbau ('building up') principle you learned in General Chemistry for writing out electron configurations, we place the two electrons in the H 2 molecule in the lowest energy molecular orbital, which is the (bonding) sigma orbital. Bonding MOs are the consequence of constructive interference between two atomic orbitals, which results in an attractive interaction and an increase in electron density between the nuclei. When two separate waves combine, they can do so with constructive interference, where the two amplitudes build up and reinforce one another, or destructive interference, where the two amplitudes cancel one another out. Remember that we are thinking here about electron behavior as wave behavior. The high-energy, antibonding sigma* orbital can be visualized as a pair of droplets, with areas of higher electron density near each nucleus and a ‘node’, (area of zero electron density) midway between the two nuclei. The bonding sigma orbital, which holds both electrons in the ground state of the molecule, is egg-shaped, encompassing the two nuclei, and with the highest likelihood of electrons being in the area between the two nuclei. The second, 's igma star' (sigma*, σ*) orbital is higher in energy than the two atomic 1 s orbitals, and is referred to as an antibonding molecular orbital. When two atomic 1 s orbitals combine in the formation of H 2, the result is two sigma ( σ) orbitals.Īccording to MO theory, one sigma orbital is lower in energy than either of the two isolated atomic 1 s orbitals –this lower sigma orbital is referred to as a bonding molecular orbital. Mathematical principles tell us that when orbitals combine, the number of orbitals before the combination takes place must equal the number of new orbitals that result from the combination – orbitals don’t just disappear! We saw this previously when we discussed hybrid orbitals: one s and three p orbitals make four sp 3 hybrids. A molecular orbital describes a region of space around two or more atoms inside which electrons are likely to be found. Recall that an atomic orbital (such as the 1s orbital of a hydrogen atom) describes a region of space around a single atom inside which electrons are likely to be found. In molecular orbital theory, we make a further statement: we say that the two atomic 1 s orbitals mathematically combine to form two new orbitals. When we described the hydrogen molecule using valence bond theory, we said that the two 1 s orbitals from each atom overlap, allowing the two electrons to be shared and thus forming a covalent bond. Let’s go back and consider again the simplest possible covalent bond: the one in molecular hydrogen (H 2). In order to understand these properties, we need to think about chemical bonding in a new way, using the ideas of molecular orbital (MO) theory. It fails to adequately account, for example, for some interesting properties of compounds that contain alternating double and single bonds. There are some areas, however, where the valence bond theory falls short.
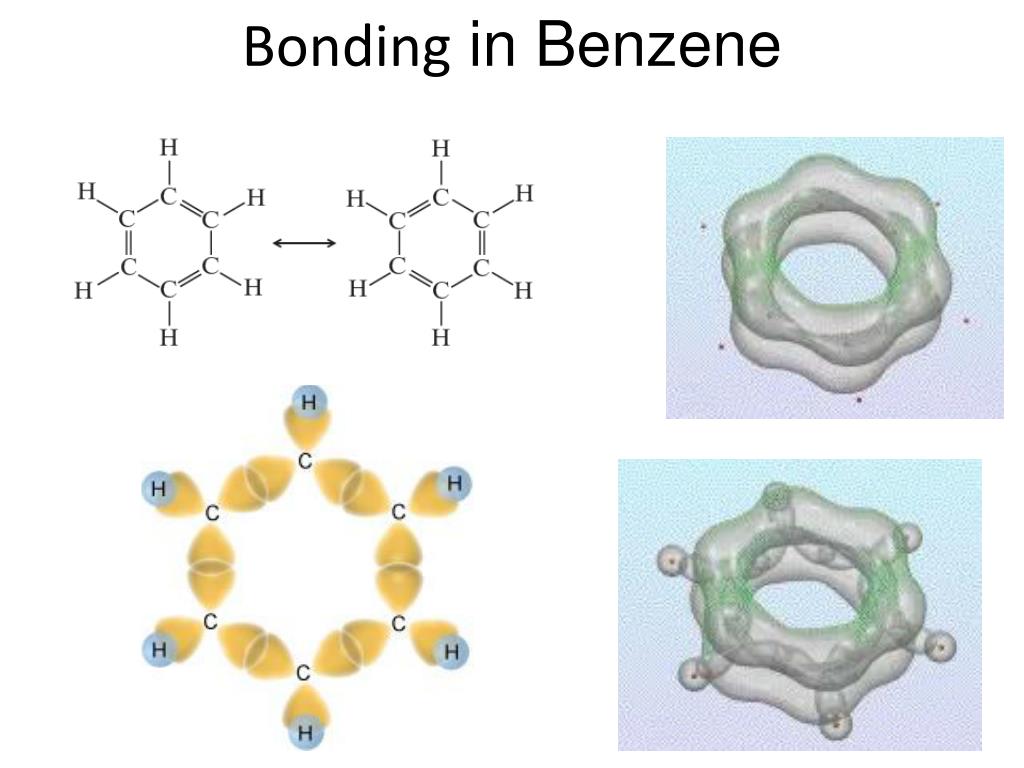
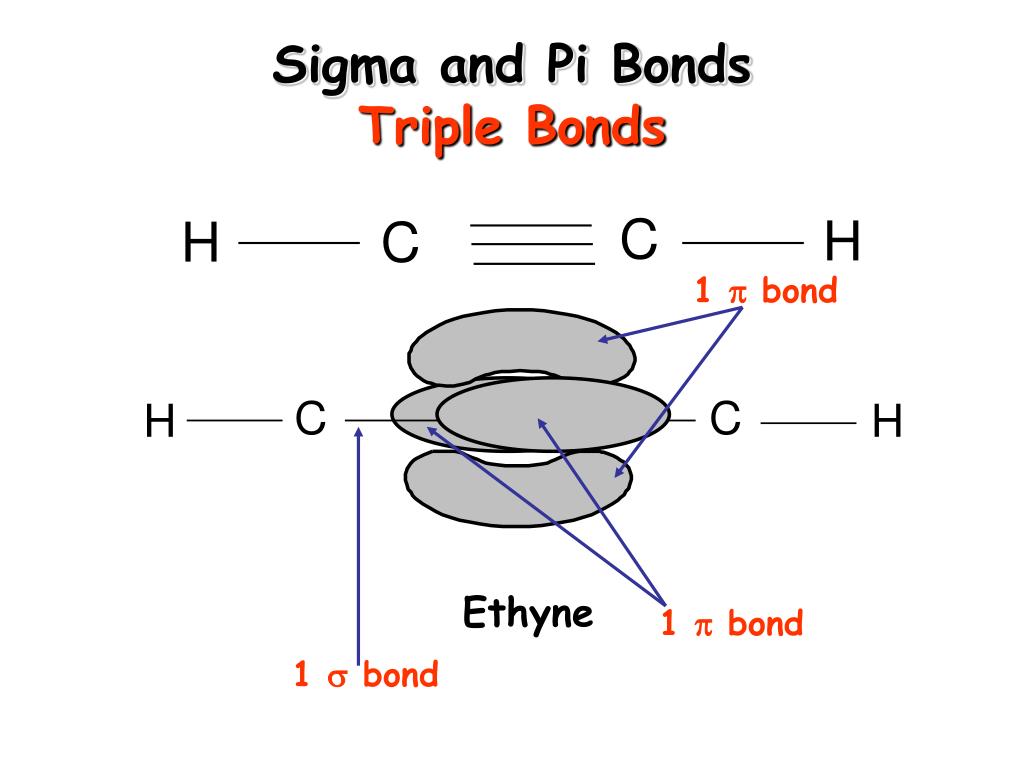
Valence bond theory does a remarkably good job at explaining the bonding geometry of many of the functional groups in organic compounds.


 0 kommentar(er)
0 kommentar(er)
